top of page

3.2.2 Group 2 Quick check
General trends of Group 2 elements
Atomic radius – increases down the group. TIB – more shells, more shielding, less attraction from nucleus to outer electrons
First ionisation energy – decreases down the group TIB – more shells, more shielding, less attraction from nucleus to outer electrons
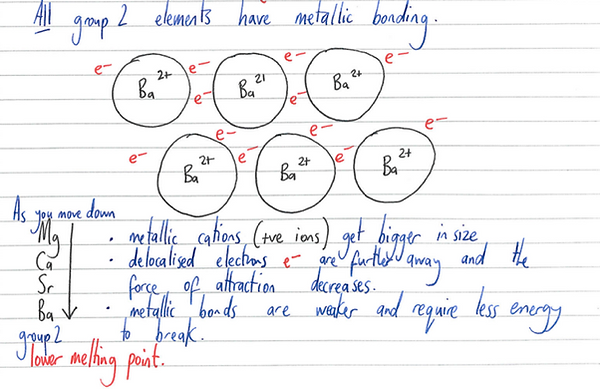
Melting points – decrease down the group. TIB – attraction between smaller cations and delocalised electrons will be stronger








bottom of page